Looking for the Right Filtration System for Your TiO₂ Production Line?
When searching for filtration solutions for titanium dioxide manufacturing, most plant engineers are not asking how the TiO₂ process works — they already know it.
What they really need to know is:
Which filtration system should be used at each critical stage to ensure stable operation, high pigment purity, and controlled operating cost?
Dongou Microfiltration supports both sulfate and chloride TiO₂ producers with field-proven solid–liquid separation systems covering waste acid recovery, TiCl₄ purification, multi-stage washing, and final finishing slurry filtration.
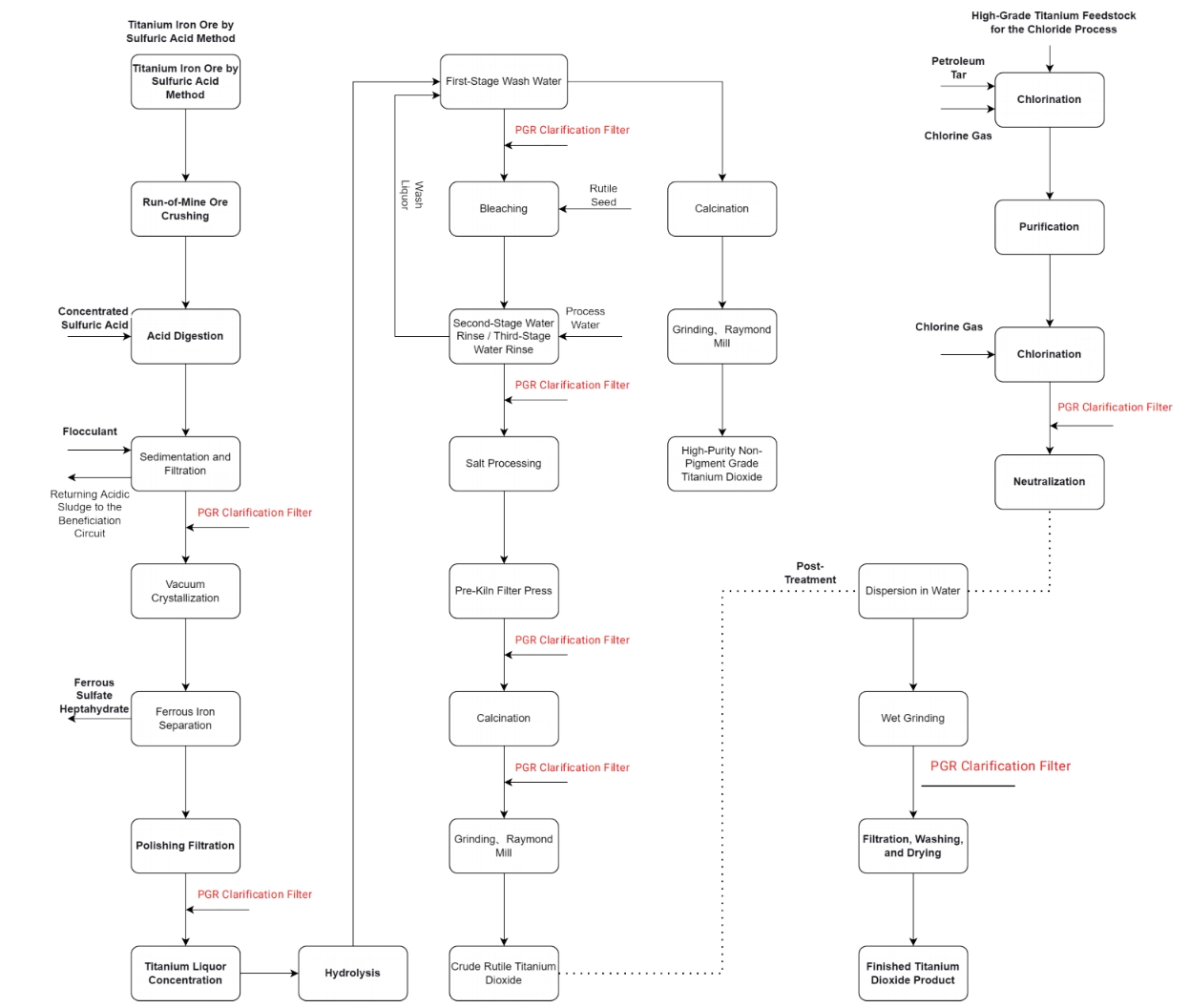
Critical Filtration Points in the Sulfate Process
A Wet-Chemistry Route Driven by Acid Digestion and Multi-Stage Filtration
The sulfate process typically starts with ilmenite or high titanium slag, which undergoes acid digestion using concentrated sulfuric acid to form a titanium sulfate solution.
How Can Waste Acid Recovery Be Improved?
After digestion and primary settling, the first critical solid-liquid separation occurs at the waste acid recovery stage. Efficient filtration here directly impacts sulfuric acid reuse, downstream wastewater load, and overall operating cost.
High-performance microfiltration at this point enables cleaner filtrate and more stable acid recovery.
How to Protect Product Whiteness Before Hydrolysis?
Following crystallization and ferrous iron separation, the process enters the metatitanic acid purification stage.
Before hydrolysis, polishing filtration removes residual fine solids, acting as a gatekeeper for downstream product whiteness and impurity control.
How to Achieve High-Purity TiO₂ After Hydrolysis?
Once acid hydrolysis converts soluble titanium species into hydrated titanium dioxide, the slurry undergoes multiple washing and filtration steps.
These washing stages are essential for removing soluble sulfates and impurities. Filtration efficiency here directly determines the final TiO₂ purity and application performance.
Critical Filtration Points in the Chloride Process
A High-Temperature Route Centered on Gas-Solid and Fine Liquid-Solid Filtration
The titanium dioxide chloride process begins with high-grade feedstock reacting with chlorine at elevated temperatures to form crude titanium tetrachloride.
Why Is Crude TiCl₄ Purification Called the “Process Lifeline”?
Immediately after chlorination, high-temperature gas-solid filtration is required to remove unreacted solids and contaminants.
This step is often described as the process “lifeline,” as crude TiCl₄ purification quality determines the optical and physical properties of the final pigment.
Post-Oxidation Slurry Filtration
After oxidation of purified TiCl₄ to form titanium dioxide particles, an initial titanium dioxide product slurry is generated.
At this point, fine solid-liquid separation concentrates the slurry and prepares it for surface treatment and finishing operations.
Final Finishing Slurry Filtration – Where Both Routes Converge
Despite their different upstream chemistries, both sulfate and chloride routes converge in the finishing section.
Here, surface treatment, desalting, and washing are followed by finishing slurry filtration, ensuring removal of residual salts before drying and calcination.
This final filtration step plays a decisive role in controlling conductivity, dispersibility, and end-use performance of titanium dioxide products.
Dongou Microfiltration – Proven Solutions for Both TiO₂ Routes
From waste acid recovery in sulfate plants to TiCl₄ purification in chloride lines, Dongou Microfiltration systems have been successfully applied across both titanium dioxide production routes.
By tailoring filtration equipment for highly acidic slurries, high-temperature gas streams, and ultrafine TiO₂ suspensions, Dongou helps producers achieve:
- Higher product purity
- Lower operating costs
- Long-term process reliability
Q: What filtration challenges are associated with waste acid recovery in TiO₂ sulfate production?
Waste acid recovery contains fine solids, dissolved iron species, and strong acidity. Filtration systems must operate reliably under corrosive conditions while preventing solids carryover that increases downstream treatment costs.
Q: How does metatitanic acid purification influence final TiO₂quality?
Residual impurities in metatitanic acid can affect particle morphology, whiteness, and electrical conductivity. Effective filtration before hydrolysis ensures cleaner feed and more consistent titanium dioxide quality.
Q: Why is TiCl₄ purification a critical filtration step in the chloride process?
In the chloride process, even trace impurities in crude TiCl₄can disrupt oxidation and degrade optical properties. Reliable purification is essential to maintain process stability and product performance.
Q: What are the key filtration requirements in the finishing slurry filtration stage?
Finishing slurry filtration must remove residual salts and chemicals without damaging surface-treated particles, directly influencing conductivity, dispersibility, and downstream application performance.
